Abstract
Introduction:
FVIII prophylaxis for hemophilia A (HA) has reduced the bleeding frequency in patients and enabled the prevention of hemophilic arthropathy and severe bleeding. However, the treatment/disease burden remains a concern, as evidenced by requiring intravenous injections two/three times a week; regular hospital visits to receive a prescription for medication, as drug delivery is not a provision in Japan; and frequent hospital visits because of complications, like bleeding. Moreover, there is no national patient registry for hemophilia in Japan, and few studies have examined the state of medical care and patient burden due to symptoms and medical practices. Herein, data from medical information databases (DBs) were assessed to investigate the state of medical care and patient burden for HA patients in Japan.
Methods:
The DBs of health insurance subscribers provided by JMDC Inc. (JMDC-DB) and of electronic medical care records provided by Real World Data Co., Ltd. (RWD-DB) were reviewed. Two DBs were used because the differences between them, such as data source for HA patients, may lead to intergroup differences in the characteristics and traceability of each patient.
The targets of analysis were HA patients (ICD-10 code: D66) prescribed FVIII concentrates, emicizumab, or bypassing agents. The definition of targets in this analysis was deemed appropriate in a separate validation study.
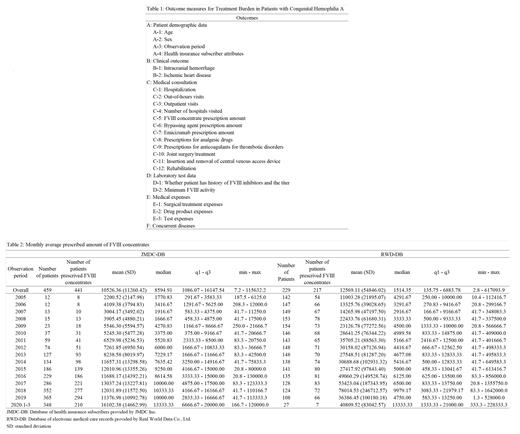
The occurrence rates of intracranial hemorrhage, ischemic heart disease, hospitalizations, emergency visits, and outpatient visits were calculated to evaluate patient burden. Further, data on the number of hospitals visited and prescribed amounts of FVIII concentrates per month were tabulated and descriptive statistics, applied. Table 1 shows the outcome measures.
Results:
The number of patients included, based on the target period and inclusion criteria, was 459 from JMDC-DB (January 2005 to March 2020) and 229 from RWD-DB (January 1985 to March 2020). Both DBs had a large proportion of patients aged 0-9 years (23.09% in JMDC-DB and 47.16% in RWD-DB) and a small proportion of patients aged ≥60 years (2.61% in JMDC-DB and 5.68% in RWD-DB).
The mean (standard deviation [SD]) and median values for the monthly prescribed amount of FVIII concentrate were 10526.36 IU (11260.42) and 8594.91 IU in JMDC-DB and 12569.11 IU (54846.02) and 1514.35 IU in RWD-DB, respectively. The yearly trends of monthly prescribed amounts of FVIII concentrate for patients in JMDC-DB were analyzed. The median values for every 3 years since 2007 were as follows: 1916.67 IU (2007), 3375.00 IU (2010), 7229.17 IU (2013), 8614.58 IU (2016), and 10000.00 IU (2019), indicating an increasing trend in recent years.
The occurrence rates (95% confidence intervals [CIs]) of intracranial hemorrhage, ischemic heart disease, and hospitalizations were 2.61% (1.36-4.52), 0.00%, and 32.68% (28.40-37.18) in JMDC-DB and 4.42% (2.14-7.99), 0.44% (0.01-2.44), and 57.08% (50.35-63.62) in RWD-DB, respectively. The age-stratified occurrence rates (95% CIs) of intracranial hemorrhage in JMDC-DB and RWD-DB were 9.43% (4.62-16.67) for 0-9 years, 1.67% (0.04-8.94) for 40-49 years, and 4.00% (0.10-20.35) for 50-59 years and 3.70% (1.02-9.21) for 0-9 years, 13.04% (2.78-33.59) for 10-19 years, 9.09% (1.12-29.16) for 30-39 years, and 6.67% (0.17-31.95) for 40-49 years, respectively. The overall occurrence rates of intracranial hemorrhage were similar in the two DBs: 2.61% (JMDC-DB) vs. 4.42% (RWD-DB).
Additional results of our analysis will be presented at the conference.
Conclusions:
The prescription of FVIII concentrates for Japanese patients with HA is increasing, probably because FVIII prophylaxis in the clinical setting has become more common. The widespread use of FVIII has many benefits for HA patients. However, there are still treatment burdens that should be considered, such as the need for several drug prescriptions and severe bleeding, such as intracranial hemorrhage.
Nagao: CHUGAI PHARMACEUTICAL CO., LTD.: Consultancy, Honoraria, Speakers Bureau; Takeda Pharmaceutical Company Limited.: Honoraria, Research Funding; Bayer Yakuhin, Ltd.: Honoraria, Research Funding; Sanofi K.K.: Honoraria; Fujimoto Pharmaceutical Corporation: Honoraria; KM Biologics Co., Ltd.: Honoraria; Pfizer Japan Inc.: Honoraria; Japan Blood Products Organization: Honoraria; Novo Nordisk Pharma Ltd.: Honoraria; CSL Behring K.K.: Honoraria. Ioka: Chugai Pharmaceutical, Co., Ltd.: Current Employment. Nakamura: Chugai Pharmaceutical Co., Ltd.: Current Employment. Murakami: Chugai Pharmaceutical Co., Ltd.: Current Employment. Makishima: Chugai Pharmaceutical, Co., Ltd.: Current Employment. Okada: Chugai Pharmaceutical Co., Ltd.: Current Employment. Sakai: Bayer Yakuhin, Ltd.: Consultancy, Speakers Bureau; Novo Nordisk Pharma Ltd.: Consultancy, Speakers Bureau; CSL Behring K.K.: Speakers Bureau; Takeda Pharmaceutical Company Limited.: Speakers Bureau; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau; Sanofi K.K.: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal